Spotlight: Real-World Evidence

Welcome to The Evidence Base Spotlight on the ‘Real-World Evidence’.
Read selected coverage from The Evidence Base covering the many and varied aspects of real-world evidence, and how it is shaping our community.
For more content on Real-World Evidence, click here.
Lead content
A new era of integrated evidence generation: Deep dive into OM1 case study spotlight from the DIA Real-World Evidence Conference 2023
In a recent presentation at the DIA Real-Word Evidence Conference, Sonja Wustrack, Managing Director of Integrated Evidence Generation at OM1, discussed strategies for optimizing evidence generation in clinical research.
Real World Radar: the first online directory of real-world data
To maximize the potential of RWE for drug development, patient care, and public health, researchers must be able to access the most relevant RWD to meet their research goals. Real World Radar, developed by Medlior Health Outcomes Research, is a digital directory of RWD that can simplify and expedite RWE research.
New guidance on best practices for peer and editorial review of RWE studies: a road map for peer-reviewed journals to drive greater transparency in RWE
Richard White, Chief Operating Officer at Oxford PharmaGenesis, discusses the best practices for the peer review of RWE research and its importance in advancing the field.
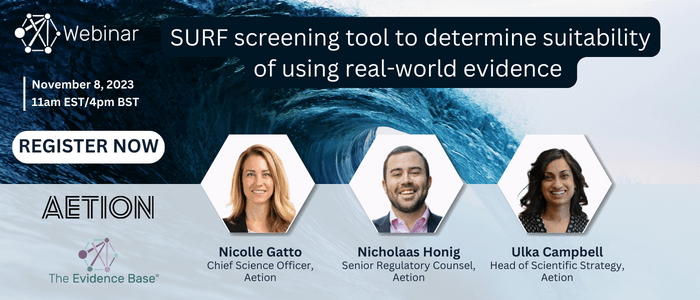
WEBINAR: SURF screening tool to determine suitability of using real-world evidence – November 8, 2023
This webinar offers insights into the regulatory acceptability of RWD and the practical application of the SURF tool, providing a concise overview of its construction and the types of evidence needed for successful RWD integration in FDA applications.
Event coverage
DIA Real-World Evidence Conference 2023 daily round-ups: Day 1
Coverage from from the DIA Real-World Evidence Conference 2023 (16–17 Oct 2023, Baltimore, MD, USA). Here, we cover the sessions from Day 1.
DIA Real-World Evidence Conference 2023 daily round-ups: Day 2
Coverage from from the DIA Real-World Evidence Conference 2023 (16–17 Oct 2023, Baltimore, MD, USA). Here, we cover the sessions from Day 2.
 1st GetReal Institute Annual Conference: Deep dive into Keynote 1 – perspectives on the evolving landscape of RWE in Europe
1st GetReal Institute Annual Conference: Deep dive into Keynote 1 – perspectives on the evolving landscape of RWE in Europe
In the first of two sessions from the 1st GetReal Institute Annual Conference looking at RWE in Europe, moderator Thomas Paulson of GSK introduced Catherine Cohet (EMA) and Marcus Guardian (EUnetHTA) to provide their perspectives on the use of RWE in Europe.
 1st GetReal Institute Annual Conference: Deep dive into Keynote 2 – Mark McClellan discusses real-world data and evidence in global contexts
1st GetReal Institute Annual Conference: Deep dive into Keynote 2 – Mark McClellan discusses real-world data and evidence in global contexts
We summarize the keynote by Mark McClellan (Duke-Margolis Center for Health Policy, USA) on ‘GetReal Faster: Advancing Real-World Data and Evidence in Global Contexts.’
Videos from the Patti’s People series
Patti Peeples speaks with Marc Berger
In this ‘Patti’s People’ episode, Patti Peeples of the The Peeples Collaborative speaks with Marc Berger, former Pharma Executive and Independent Consultant for real-world data and real-world evidence.
Patti Peeples speaks with Nancy A. Dreyer
In this ‘Patti’s People’ episode, Patti Peeples of the The Peeples Collaborative speaks with Nancy A. Dreyer, Adjunct Professor of Epidemiology at the University of North Carolina, and Chief Scientific Officer, IQVIA Real-World Solutions, Retired.
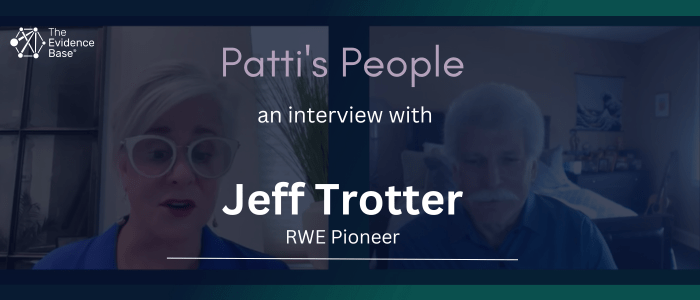
Patti Peeples speaks with Jeff Trotter
In this ‘Patti’s People’ episode, Patti Peeples of the The Peeples Collaborative speaks with RWE Pioneer, Jeff Trotter.
From our Expert Insights
Ensuring rigor and reliability in real-world evidence: an interview with Shirley Wang, Brigham and Women’s Hospital, Harvard Medical School
Here Shirley Wang (Brigham and Women’s Hospital, Harvard Medical School) discusses several collaborative efforts being carried out aimed at ensuring rigor and reliability in RWE.
Using real-world data from social listening to generate patient experience insights: an interview with Mathias Leddin, Roche
In this interview, Mathias Leddin (Roche) discusses the progress that is being made in harnessing RWD from social listening and outlines the challenges and potential solutions that are needed to advance the field.
 NICE’s real-world evidence framework in action: an interview with Pall Jonsson and Stephen Duffield, NICE, UK
NICE’s real-world evidence framework in action: an interview with Pall Jonsson and Stephen Duffield, NICE, UK
One year on from the launch of the ‘NICE real-world evidence framework’, we speak with Pall Jonsson and Stephen Duffield of NICE to learn how they have implemented this framework.
Peek Behind the Paper: Availability of comparative RWE in Medicare patients – implications for CMS drug price negotiations
In this interview we speak with Ashley Jaksa, Market Access Scientific Strategy Lead and Pat Arena, Director, Epidemiology at Aetion about their recent scoping review.
Using artificial intelligence tools to develop plain language summaries of HEOR and RWE publications: an interview with David McMinn, Sorcero
David McMinn, Lay Summaries Practice Leader at Sorcero, discusses the importance of PLS in communicating medical research and describes the role of artificial intelligence as a tool to create PLS of complex research.
Impact of the Inflation Reduction Act on real-world evidence: an interview with Amanda Cole, Office of Health Economics
Amanda Cole, Associate Director at the Office of Health Economics about the opportunity for the IRA to advance RWE in the US.
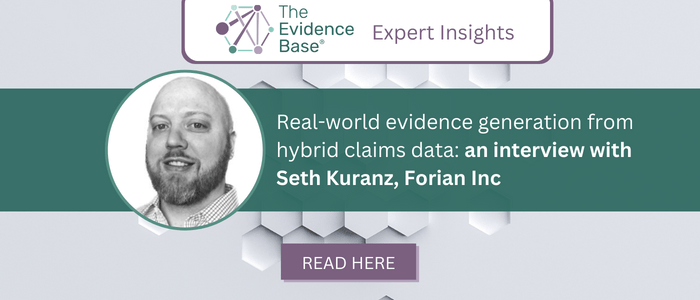
Real-world evidence generation from hybrid claims data: an interview with Seth Kuranz, Forian Inc
Seth Kuranz, RWE Principal, Forian, explores the opportunities for RWE generation using hybrid claims data.
Role of patients in the generation and communication of real-world studies: an interview with Catherine Bottomley
In this interview we speak with Catherine Bottomley about how patients can partner with companies on all aspects of RWE.
Transferability of real-world evidence methods and patient engagement practices in health technology assessment from Western to Central and Eastern European countries: an interview with Bertalan Németh
Bertalan Németh (Syreon Research Institute) discusses the ‘Transferability and dissemination’ work package from HTx, which has been tasked with understanding the transferability of knowledge and methods gained to Central and Eastern European countries.
Get in touch to discuss the opportunities available to share your expertise, contribute to this theme or to become a theme sponsor.