EMA publishes new guidance on real-world evidence generation service

The European Medicines Agency (EMA) has released new guidance detailing the types of studies that can be performed for RWE generation and how they can support RWD studies in the context of regulatory decision-making.
Real-world evidence (RWE) plays an increasingly vital role in informing regulatory decision-making regarding human medicines. April 2024 saw the publication of a new document outlining the EMA’s support for regulatory decision-making using RWE.
Within the EMA, the Data Analytics and Methods Task Force‘s Real-World Evidence Team (TDA-RWE) is responsible for providing the RWE generation service. Through patient electronic medical records, TDA-RWE has access to real-world data (RWD) both directly and indirectly. This access enables them to support regulatory evaluations and decision-making processes. EMA then offers a service to generate and deliver RWE to various stakeholders, including scientific committees, national competent authorities (NCAs), EMA functions, and other EU decision-makers and partner organizations. Requests from organizations including academia, contract research organizations and pharmaceutical companies are outside the scope of the guidance and will not be considered.
As the EMA note, RWD serves as a complementary source of evidence, filling knowledge gaps and providing independent and transparent insights. EMA tailors analyzes to address specific questions raised by committees or requesters, potentially expediting evidence generation and avoiding procedural steps required for industry-sponsored studies. It also enables the study of multiple products of the same class, reducing duplication and inefficiencies common in industry-led studies.
Three main pathways are available for RWE generation:
- The Data Analysis and Real-World Interrogation Network (DARWIN EU®)
- Studies using in-house electronic health databases
- Studies procured through EMA framework contracts
These pathways support a range of studies, from simple assessments to addressing complex issues. Simple studies include estimating the prevalence of diagnosed conditions, incidence rates, drug utilization patterns, and event rates following medication exposure. In DARWIN EU, these studies are expected to be carried out with a generic protocol and are referred to as off-the-shelf studies. Complex studies involve causal inference, complex coding or phenotyping, and advanced analytical approaches such as clinical prediction models. Regardless of complexity, studies conducted via DARWIN EU® can be repeated regularly to track trends over time.
Committee members, assessors, NCA representatives, EMA staff, and other EU decision-makers with whom EMA has agreed to provide support, are encouraged to submit research questions. These inquiries can aid pharmaceutical evaluation at all stages. The figure below outlines three key areas where RWE supports regulatory decisions:
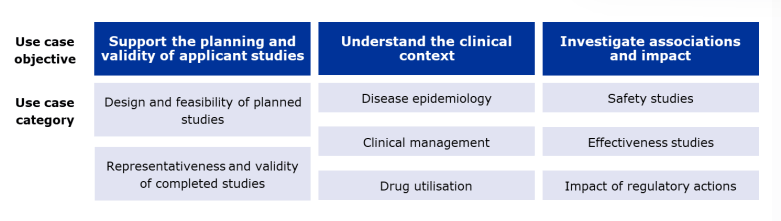
Real-world evidence provided by EMA: Support for regulatory decision-making © European Medicines Agency, 2024
TDA-RWE comprises epidemiologists, statisticians, and data analysts with expertise in analyzing RWD for medicines-related research questions. EMA ensures that the generated evidence meets exacting standards of validity and reliability, maintaining a rigorous review process for protocols and analysis plans.
Requests for RWD studies are accepted from members of the European Medicines Regulatory Network (EMRN) and other specified bodies and institutions. These requests undergo a structured process, involving feasibility assessments and close collaboration with requesters to refine research questions and study designs.
Want regular updates on the latest real-world evidence news straight to your inbox? Become a member on The Evidence Base® today>>>