Spotlight: ATMPs, Orphan Drugs & Rare Diseases

Welcome to The Evidence Base Spotlight on the ‘ATMPs, Orphan Drugs & Rare Diseases’.
Read selected coverage from The Evidence Base covering the many ways in which our industry is supporting development in this important area.
For more content, click here.
Featured content
 ATMPs: What are they and why does real-world evidence matter?
ATMPs: What are they and why does real-world evidence matter?
In this summary article, we explore advanced therapy medicinal products (ATMPs) – taking a look at what they are, their role in the context of rare diseases and the place of real-world evidence.
Panel Discussion – Decision uncertainty in HTA: what next after managed access?
Outcomes-based payment models (OBPs) offer the opportunity to minimize the uncertainty associated with the reimbursement of new therapies, particularly those that are more innovative.
Demonstrating the value of highly innovative technologies with real-world evidence: an interview with Karen Facey, RWE4Decisions
In this interview, we speak with Karen Facey (University of Edinburgh, UK and RWE4Decisions Facilitator) to learn more about the work of the multi-stakeholder initiative, RWE4Decisions.
Spotlight: Rare Diseases and Innovative Therapies
Catch up on this virtual event, including a mix of expert interviews, a panel discussion on accelerated regulatory approval, and a Fireside chat with Jean-Christophe Novelli, who discusses the human impact of rare diseases from his first-hand experiences.
Identifying hidden patients with rare diseases in real-world research: a case study
In this Editorial, Justin Chen and Victoria Divino (IQVIA), discuss a case study describing an innovative approach and solution to identify ‘hard-to-find’ (e.g., rare diseases, lack of consensus on available diagnosis codes) patients in real-world databases.
Understanding External Control Arms: An Interview with Dr Lewis Carpenter, Head of Real-World Evidence, Arcturis
Laura Dormer, Editor of The Evidence Base, speaks with Dr Lewis Carpenter, Head of Real-World Evidence, Arcturis, about External Control Arms.
Rare disease and the lessons learned from the COVID-19 pandemic – Rebecca Stewart
Rebecca is RareRevolution Magazine’s co-founder. With a passion for meeting individuals and building networks, Rebecca has channeled a community approach to realizing meaningful, accessible resources and the power of education through compelling story telling.
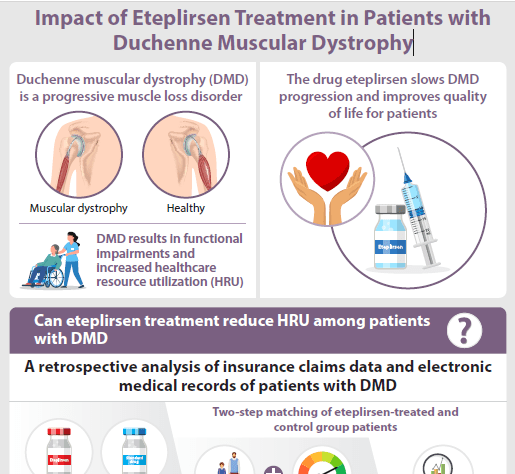 Infographic: Impact of eteplirsen treatment in patients with Duchenne muscular dystrophy
Infographic: Impact of eteplirsen treatment in patients with Duchenne muscular dystrophy
This infographic is a summary of an article originally published in the Journal of Comparative Effectiveness Research. The article is called ‘Real-world evidence of eteplirsen treatment effects in patients with Duchenne muscular dystrophy in the USA’ and can be read here.
Get in touch to discuss the opportunities available to share your expertise, contribute to this theme or to become a theme sponsor.