Duke-Margolis Center for Health Policy releases International Harmonization of Real-World Evidence Standards Dashboard

With real-world evidence (RWE) playing an increasingly important role in regulatory decision-making for medical products, there is an unmet need to provide a centralized resource for stakeholders to stay up-to-date with the latest guidance from international bodies. To achieve this goal, Duke-Margolis Center for Health Policy has released the International Harmonization of Real World Evidence Standards Dashboard, providing a navigable tool displaying the list of resources available from global regulatory agencies.
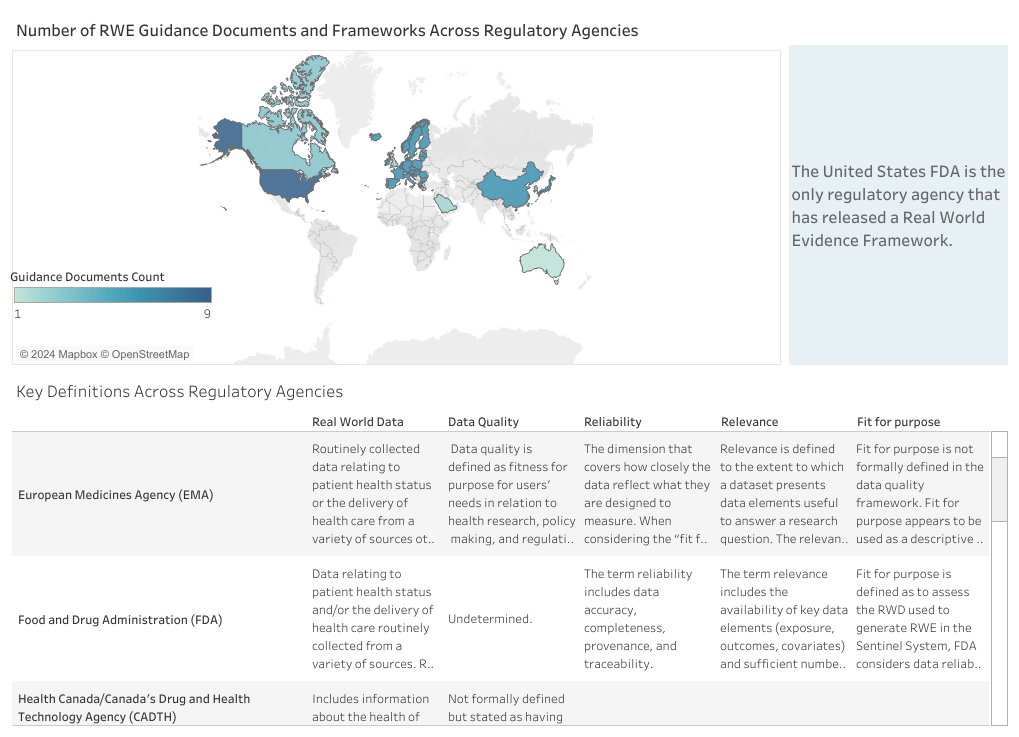
The International Harmonization of Real World Evidence Standards Dashboard has been developed by the Duke-Margolis RWE policy research team, which includes representatives from industry, data companies, payers, research groups, providers, patient networks, regulatory affairs, law, health and data science, and policy. Commenting on Linkedin, Rachele Hendricks-Sturrup (Research Director Real-World Evidence, Duke-Margolis Center for Health Policy) explained:
The Duke-Margolis RWE policy research team and I have given tremendous effort in partnership with our RWE Collaborative to develop the Dashboard, with the goal of helping internationally-focused RWE policy stakeholders track, in one place, relevant and timely regulatory guidance, frameworks, international harmonization documents, and more.
 Learn more about the guidelines and resources for the use of RWE in regulatory decision making in the infographic ‘Global landscape for RWD and RWE initiatives in regulatory decision making‘ created by The Evidence Base®.
Learn more about the guidelines and resources for the use of RWE in regulatory decision making in the infographic ‘Global landscape for RWD and RWE initiatives in regulatory decision making‘ created by The Evidence Base®.
Want regular updates on the latest real-world evidence news straight to your inbox? Become a member on The Evidence Base® today>>>






