Pembrolizumab plus chemotherapy for first-line treatment of lung cancer: a network meta-analysis
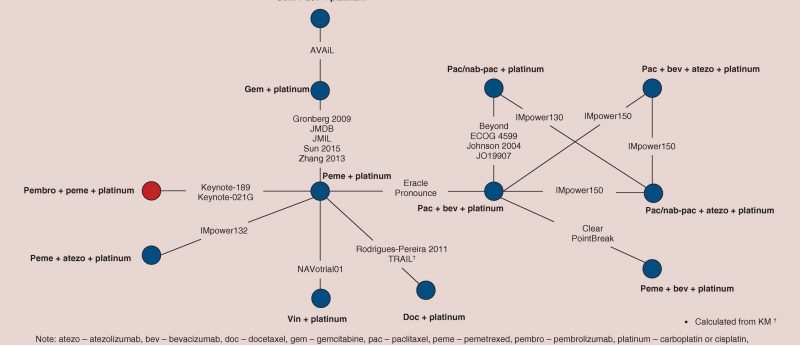
Read an excerpt from a systematic review evaluating the efficiency of pembrolizumab + pemetrexed + platinum relative to other regimens in metastatic nonsquamous non-small-cell lung cancer (NSq-NSCLC).
The introduction of immunotherapy has led to a rapid transformation in the treatment of metastatic non-small-cell lung cancer (NSCLC). The PD-1/PD-L1 inhibitors, nivolumab, pembrolizumab and atezolizumab were first approved for advanced NSCLC in previously treated disease, based on significantly improved overall survival (OS) compared with docetaxel. Pembrolizumab monotherapy was subsequently approved for the first-line treatment of metastatic NSCLC whose tumors have high PD-L1 expression (tumor proportion score [TPS] ≥50%) and with no EGFR mutation or ALK gene rearrangement, based on significantly improved OS compared with platinum-based doublet chemotherapy.
In this systematic review, Andrew M Frederickson, Stella Arndorfer, Ina Zhang, Maria Lorenzi (all Precision Xtract; CA, USA), Ralph Insinga, Ashwini Arunachalam, Thomas A Burke (all Merck & Co.; NJ, USA) and George R Simon (The University of Texas MD Anderson Cancer Center; TX, USA) assess the relative efficacy of pembrolizumab + pemetrexed + platinum to additional competing interventions, including other immunotherapy-based regimens for the first-line treatment of metastatic nonsquamous NSCLC patients without tumor EGFR mutation or ALK translocation by means of a network meta-analysis (NMA).
