Patient characteristics and bleeding events in nonvalvular atrial fibrillation patients treated with apixaban or vitamin K antagonists: real-world evidence from Italian administrative databases
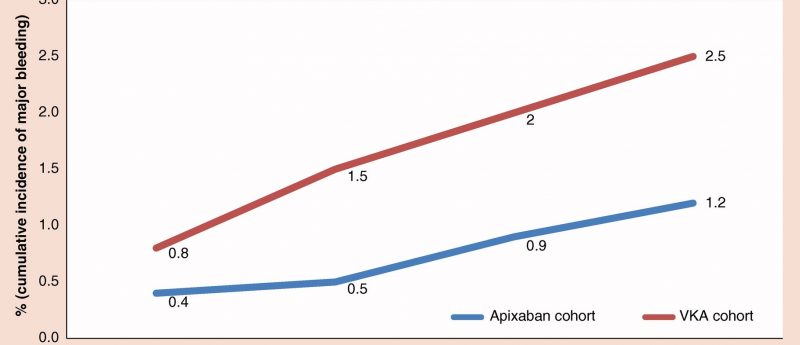
In this article, the authors evaluate the risk of major bleeding among two cohorts of nonvalvular atrial fibrillation patients newly initiating a vitamin K antagonist (VKA) or apixaban.
International guidelines on atrial fibrillation (AF) management suggest that important advances in pharmacologic therapy have occurred in the last decade, particularly with regard to oral anticoagulants (OACs) for stroke prevention in patients with nonvalvular AF (NVAF).
OACs currently available in Italy for NVAF include vitamin K antagonists (VKAs) and novel direct OACs (NOACs), such as dabigatran, rivaroxaban, apixaban and edoxaban. The use of any OAC is guided by international and national criteria and described in professional guidelines. This guidance balances the risk of stroke as well as bleeding using OAC therapy and as such OAC treatment is recommended for high-stroke risk patients (i.e., those with ≥2 stroke risk factors) and not recommended to AF patients without additional stroke risk factors.
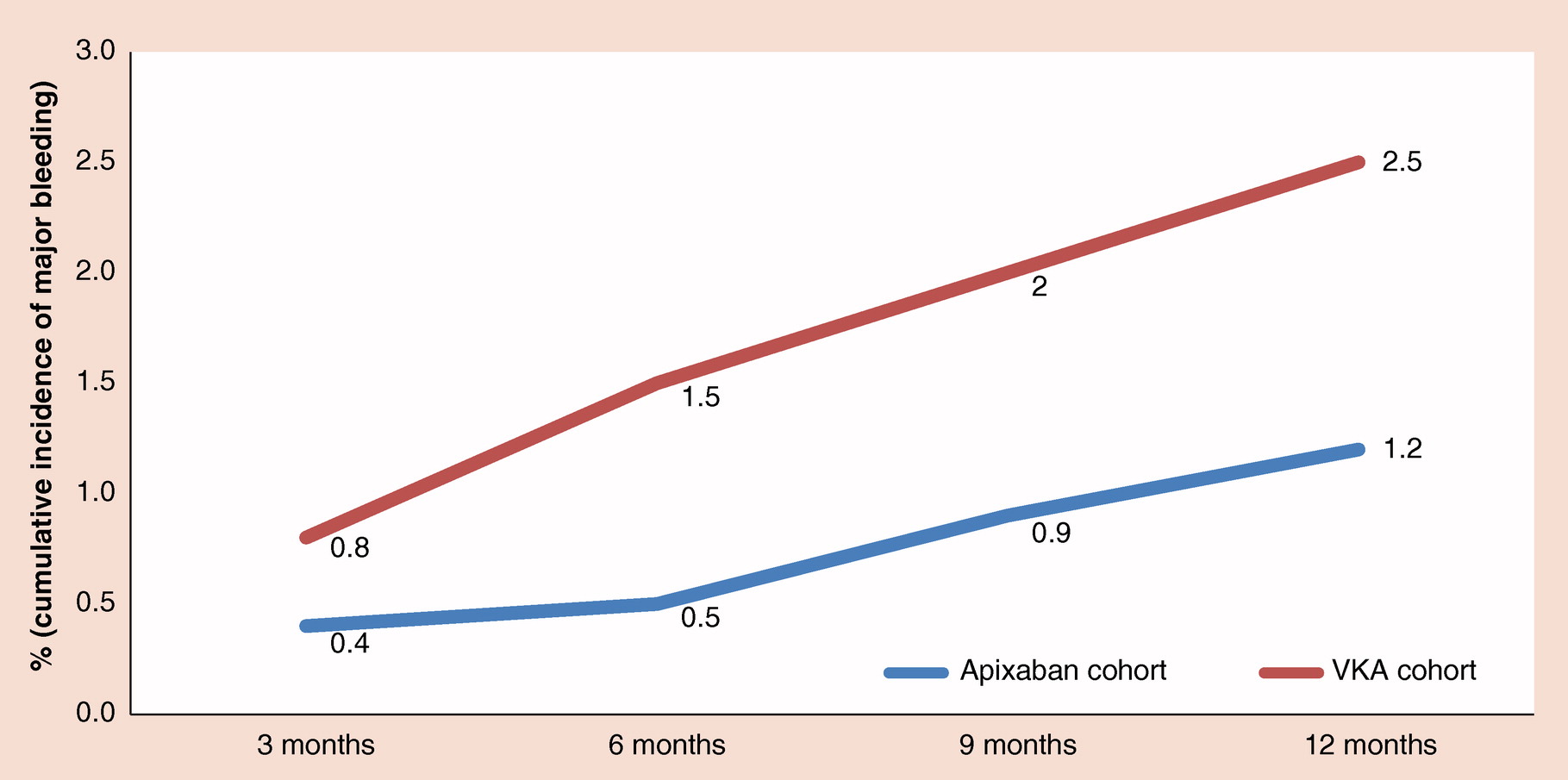
In this retrospective study, researchers from Bristol-Myers Squibb (UK and Rome, Italy) evaluate the risk of major bleeding among two cohorts of nonvalvular atrial fibrillation patients newly initiating a vitamin K antagonist (VKA) or apixaban in a real-world setting in Italy.
